Permanent birth
control in clinical trial
The lack of innovation for permanent birth control over the last half century leaves women struggling for viable options.
Birth Control
The only permanent birth control option available to women around the world is surgical tubal ligation, which was first performed in the 1880s. Although effective, surgery is associated with scarring from incisions, increased safety risks, recovery time and may not be a suitable option. The demand for accessible contraceptive options, particularly permanent, continues to rise.
More than 13 million women no longer intend to have children in the U.S.1,2
1.2 million women choose tubal ligation every year in the U.S.2,4
- Jones et. al, Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. National health statistics reports; no 60. Hyattsville, MD: National Center for Health Statistics. 2012.
- Daniels et. al, Current contraceptive status among women aged 15–49: United States, 2017–2019. NCHS Data Brief, no 388. Hyattsville, MD: National Center for Health Statistics. 2020.
- National health statistics reports; no 60. Hyattsville, MD: National Center for Health Statistics. 2012.
- Martinez GM. Receipt of family planning services in the United States: 2022–2023. NCHS Data Brief, no 520. Hyattsville, MD: National Center for Health Statistics. 2024. DOI: https://dx.doi.org/10.15620/cdc/169629
FemBloc Permanent Birth Control
— Now Approved in Europe —

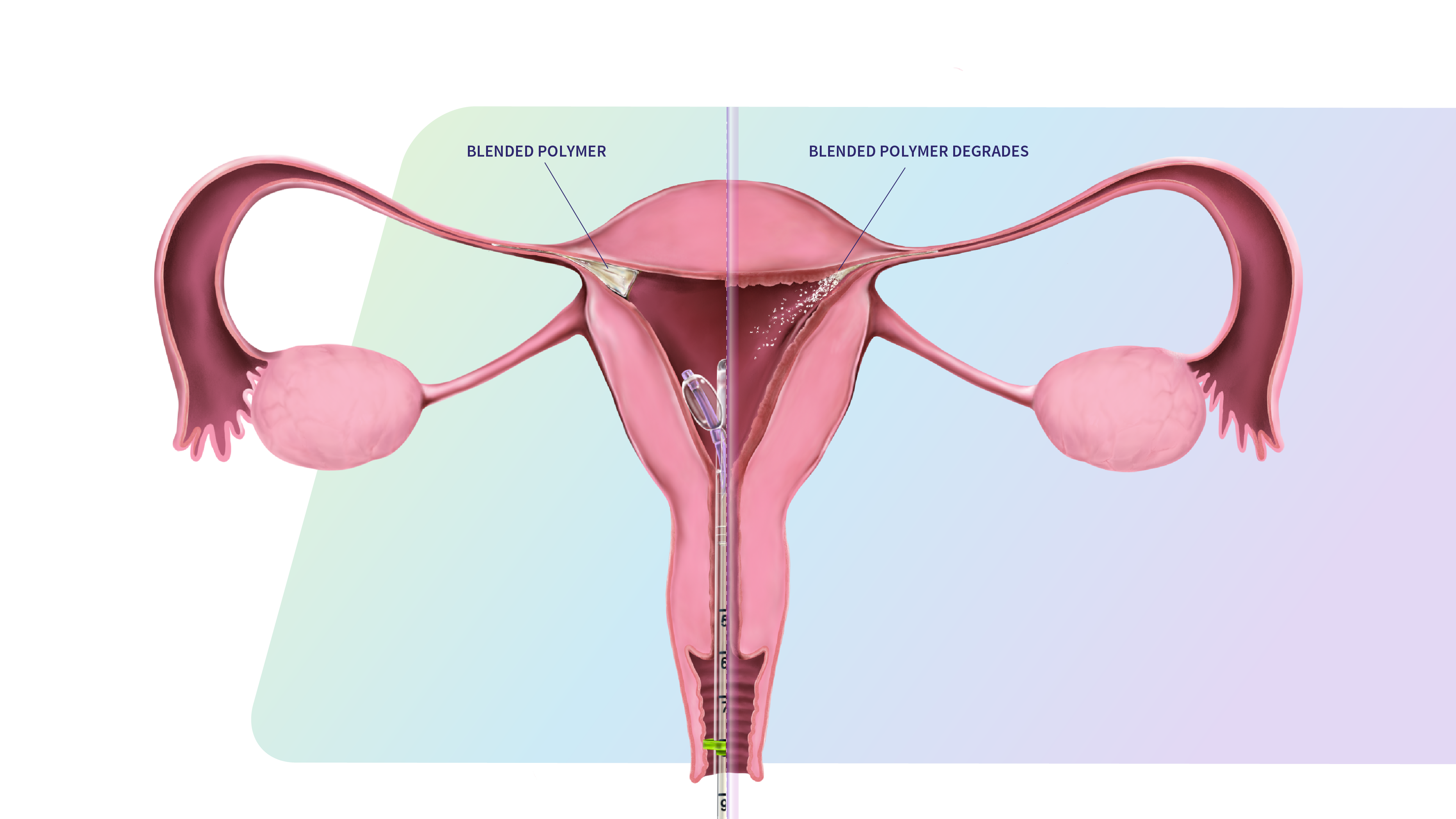
FemBloc is a revolutionary first-of-its-kind non-surgical permanent birth control approach, designed to precisely deliver our proprietary synthetic tissue adhesive (called Blended Polymer) into both fallopian tubes simultaneously. Over time, the blended polymer fully degrades and produces nonfunctional scar tissue to permanently block the fallopian tubes. In contrast to historic surgical sterilization, the FemBloc approach offers a non-surgical, more accessible in-office alternative with fewer risks, contraindications, and substantially lower cost. Confirmation of procedure success can be achieved after 90 days, offering certainty unavailable with other options.
FemBloc is now approved in Europe.
Clinical Data
Trial Design:
Prospective, multi-center, unblinded clinical trials with historical control. Conducted under FDA Investigational Device Exemptions (IDEs). NCT03067272, NCT03433911, and NCT04273594.
Trial Results5:
(for subjects who met trial eligibility and were determined bilaterally blocked after confirmation test)
Primary Endpoint
- Pregnancy rate was 0% (n=51)
SAFETY
- Adverse events were consistent with those typically observed for intrauterine transcervical procedures, with no on-going safety concerns through five years.
There were no reports of serious adverse events (n=0/229).
Participants are currently being enrolled in the FINALE pivotal clinical trial (NCT05977751) for U.S. approval.
- Liu, J. H., Blumenthal, P. D., Castano, P. M., Chudnoff, S. C., Gawron, L. M., Johnstone, E. B., Lee-Sepsick, K. (2025). FemBloc Non-Surgical Permanent Contraception for Occlusion of The Fallopian Tubes. J Gynecol Reprod Med, 9(1), 01-12. doi: 10.33140/ JGRM.09.01.05.
- Gariepy AM, Lewis C, Zuckerman D, et al. Comparative Effectiveness of Hysteroscopic and Laparoscopic Sterilization for Women: A Retrospective Cohort Study. Fertility and Sterility, 2022, 117(6):1322-1331. Doi: 10.1016/j.fertnstert.2022.03.001.
Clinical Trial Status
for U.S. FDA approval
How it Works
Blended Polymer delivery
- Delivery device is placed through cervix into uterine cavity
- Balloon catheters are advanced and inflated
- Blended Polymer is delivered into each fallopian tube
Blended Polymer transformation
- Delivery device is removed after blended polymer delivery
- Blended polymer transitions to solid from liquid with local tissue contact
Blended Polymer degradation
- Blended Polymer is designed to degrade over time and completely expel naturally
Confirmation of success
- Small section of each fallopian tube is closed with nonfunctional scar tissue
- Ultrasound test confirms blocked tubes for permanent birth control